 News & Events
News & Events
 PHIRDA
PHIRDA  2025.12.22
2025.12.22
 148
148
From November 15 to 19, 2025, the ICH Meeting was successfully held in Singapore. During the meeting, ICH continued to expand National Agency for Food and Drug Administration and Control (NAFDAC), Nigeria and South African Health Products Regulatory Authority (SAHPRA), South Africa as new ICH Members; in addition two new Observers: Dirección General de Medicamentos, Alimentos y Productos Sanitarios (DIGEMAPS), Dominican Republic and Food and Drug Administration (Philippine FDA), Philippines, bringing ICH to a total of 25 Members and 41 Observers.
During the ICH Meeting, the MedDRA Steering Committee and 12 ICH Expert Working Groups (EWGs) convened to advance the international harmonization of guidelines. Five Task Force Leads from China Pharmaceutical Innovation and Research Development Association (PhIRDA) were invited to attend in person. On behalf of the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) Task Forces,they participated in discussions on the revision of relevant ICH guidelines, with details as below:
MedDRA Steering Committee
Lead: GUO Xiaojun, Vice President, Drug Safety and Pharmacovigilance of Hutchmed
The MedDRA Steering Committee is responsible for managing support and facilitate the maintenance, development and dissemination of the Medical Dictionary for Regulatory Activities (MedDRA). MedDRA is updated twice a year; the latest version (28.1) is available in 27 languages, including the newly added Norwegian, Slovak, and Slovenian. To better support user training, the Maintenance and Support Services Organization (MSSO) launched the MedDRA Learning Management System (LMS) in early 2025. MedDRA users can earn certificates and badges through online learning. Additionally, a new course Coding Manufacturing and Quality System Issues with MedDRA has been launched on the MedDRA.org website.
Q6(R1): Revision of the Specifications Guidelines
Lead: AN Zhenming, Head of Institute of Biotechnology Development of Qilu Pharmaceutical
Prior to this ICH Meeting, the ICH Q6 EWG completed drafting the full guideline and conducted an internal review. This meeting held targeted discussions on the key issues raised during the review, reaching consensus or clarifying directions for revisions. Additionally, the EWG discussed and optimized the overall structure of the guideline; while further enhancing logical clarity, the revised structure will also provide more guidance for biologics, chemical drugs, and other innovative drug formats. Following the meeting, the ICH Q6 EWG will revise, supplement and refine the guideline draft in accordance with the discussions and resolutions made at this Meeting, and the draft will be finalized prior to constituents’ review planned for March to April 2026.
The ICH Q6 EWG also conduct interaction with the ICH S13 and Q1 EWGs to enhance consistency across respective guidelines.
S13: Nonclinical Safety Studies for Oligonucleotide-based Therapeutics
Lead: GONG Xuelian, Director of Toxicology and Pharmacokinetics, Shanghai Research Institute of Jingxin Pharmaceutical
The ICH S13 EWG completed the drafting and submission of the concept paper in November 2024, and finalized the guideline outline in June 2025. The objective of this meeting was to advance the harmonization and drafting of the main body of the guideline. During the meeting, cross-team meetings were conducted with the the ICH E14/S7B and Q6 EWGs, establishing a firm basis for the harmonization and improvement of guidelines across different fields.
M11: Clinical electronic Structured Harmonized Protocol (CeSHarP)
Lead: LI Wenbin, Chair of Cancer Center of Beijing Tiantan Hospital, Capital Medical University
This meeting focused on promoting the implementation of the ICH M11 Guideline, conducting in-depth discussions on core topics including the finalization of deliverables, the progress on training development, and the establishment of change management mechanisms. The meeting yielded several key outcomes: the formal approval of CeSHarP Agreement deliverables was completed; the delivery plan for 8 core training modules was reviewed and aligned; and a plan for the review, approval and application of training modules in 2026 was drafted.
M18: Framework for Determining Utility of Comparative Efficacy Studies in Biosimilar Development Programs
Lead:DOU Changlin, President of R&D, Chief Operating Officer and Executive Board Member of Boan Biotechnology
At this meeting, the ICH M18 EWG discussed and finalized the Concept Paper of Framework for Determining the Utility of Comparative Efficacy Studies in Biosimilar Development Programs, and submitted it to the ICH Assembly for approval. The ICH M18 EWG has also initiated discussions on the structure and content of the guideline, started drafting of M18 guideline and created a preliminary work plan. It was agreed to meet in-person in May 2026 in Rio, Brazil to review progress of M18 guideline development.

Group photo of IFPMA delegates (2nd row, from left to right: DOU
Changlin,
AN Zhenming, LI Wenbin, GONG Xuelian; 2nd row, 2nd from left: GUO Xiaojun)

M18 EWG (3rd row, 1st from right: DOU Changlin)

Q6 EWG (3rd row, 5th from left: AN Zhenming)

MedDRA Steering Committee (1st row, 2nd from right: GUO Xiaojun)
During the ICH Meeting, experts from PhIRDA also held in-depth exchanges with experts from China’s National Medical Products Administration (NMPA), European Commission (EC), U.S. Food and Drug Administration (FDA), Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) and member institutions of IFPMA. As the leads of the relevant IFPMA Task Forces, the five experts fulfilled their duties conscientiously: they led and organized Task Force meetings, coordinated and advanced related work, attended the ICH Meeting in person, and participated extensively in all aspects of guideline formulation and revision. These efforts helped build a bridge for dialogue between the global pharmaceutical industry and regulatory bodies, promoted the alignment of China’s industry standards with international norms, enhanced China’s participation and influence in the global rule-making process for pharmaceuticals, and contributed Chinese expertise to global pharmaceutical innovation.
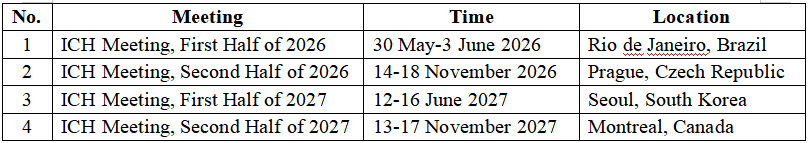
As of November 2025, PhIRDA has nominated 70 experts (including 13 Leads and 10 Alternates) to 32 IFPMA ICH EWGs, accounting for 43% of IFPMA’s global expert pool. The meeting schedule for the next two years was also announced. PhIRDA will continue to actively coordinate expert participation, engage deeply in the development and revision of international regulations, represent the voice of the Chinese pharmaceutical industry, and contribute to the high-quality development of the global pharmaceutical sector.

 News & Events
News & Events
 PHIRDA
PHIRDA  2026-01-19
2026-01-19
 56
56

 News & Events
News & Events
 PHIRDA
PHIRDA  2025-12-30
2025-12-30
 110
110

 News & Events
News & Events
 PHIRDA
PHIRDA  2025-12-22
2025-12-22
 148
148