 News & Events
News & Events
 2024.11.20
2024.11.20
 933
933
The 2024 ICH Assembly was held in Montreal, Canada, from November 2 to 6. At the Assembly, the Center for Pharmaceutical Products Safety (CPPS) under the Ministry of Health of the Republic of Uzbekistan, the General Directorate of Medicines, Supplies and Drugs (DIGEMID) of Peru, and the Thailand Food and Drug Administration (Thai FDA) were officially joined as new ICH Observers, making ICH a total of 23 Members and 38 Observers.
Representatives from 11 Expert Working Groups (EWGs) and 1 Discussion Group attended the meeting, and significant progress was made on several guidelines. The Concept Paper for the new ICH S13 Guideline on “Nonclinical Safety Studies for Oligonucleotide-based Therapeutics” was adopted by the ICH Management Committee. The Draft ICH E6(R3) Annex 2 Guideline on “Good Clinical Practice (GCP)” and the Draft ICH M15 Guideline on “General Principles for Model-Informed Drug Development (MIDD)” was endorsed by the ICH Assembly and entered Public Consultations stage. The ICH M13A Guideline on “Bioequivalence for Immediate-Release Solid Oral Dosage Forms” and the ICH E11A Guideline on “Pediatric Extrapolation” reached Step 4 and officially entered the implementation phase. These important advancements in the guidelines provide a solid foundation for further global coordination in drug development and regulatory affairs, promoting international regulatory harmonization.
At the Assembly, three experts recommended by China Pharmaceutical Innovation and Research Development Association (PhIRDA), working as the Lead Experts of the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) ICH Expert Working Groups, were invited to attend onsite. They engaged in the revision discussions of the guidelines of the ICH M11, Q6(R1), and E2D(R1) EWGs. The list of experts is as follows:
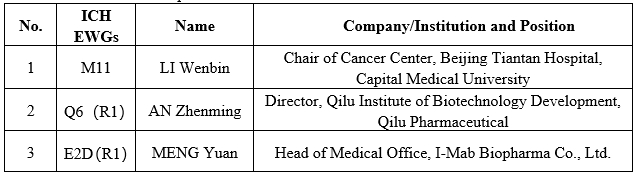

IFPMA Representatives (From right to left: MENG Yuan, AN Zhenming, LI Wenbin)
The three experts stated that during the ICH Assembly, several important discussions were conducted and significant consensus was reached. The M11 EWG reviewed and revised hundreds of terms and definitions potentially involved in clinical trials. The EWG reached an agreement on revisions to templates and technical specifications and discussed training matters related to Multi-Regional Clinical Trials (MRCTs) to ensure the correct understanding and implementation of the M11 Guideline globally.
The Q6(R1) EWG, in alignment with industry development and technological advancements, analyzed the original guideline across three sessions: general principles, chemical drugs, and biologics. This EWG held preliminary discussions on concepts and statements that needed updating and proposed a framework for the new guideline. In the next phase, the group will begin drafting based on the consensus reached during the meeting. The E2D(R1) EWG completed the Step 2 of collecting comments in July 2024, receiving over 450 suggestions globally. The EWG is currently reviewing each suggestion. The Step 3, involving approval by regulatory authorities in various countries, is expected to be completed by April 2025.
The three experts fulfilled their responsibilities with dedication. As Lead Experts, they not only organize regular working group meetings but also personally attend the annual ICH Assembly, deeply engaging in the development and revision of guidelines. They build a bridge for communication between China’s pharmaceutical industry, international regulatory bodies, and the global industrial community. Their efforts have promoted the high-quality development of China’s biopharmaceutical industry, accelerated its alignment with international standards, shortened drug development cycles, and contributed actively to advancing global pharmaceutical innovation.

M11 Expert Working Group Meeting (LI Wenbin)

E2D (R1) Expert Working Group Representatives (Front Row, Fifth from Left: MENG Yuan)

Q6 (R1) Expert Working Group Representatives (Fourth Row from the Right, First on the Left: AN Zhenming)
As of November 2024, PhIRDA has nominated 55 experts (including 11 Lead Experts, 9 Alternates) to 29 IFPMA ICH EWGs, accounting for 37% of the global experts of IFPMA. PhIRDA has proactively nominated experts to participate in the IFPMA ICH WGs, ensuring comprehensive involvement of Chinese experts in the development and revision of international guidelines. On behalf of PhIRDA and China’s pharmaceutical industry, these experts made the industry’s voice heard, and promoted the implementation of ICH Guidelines in China smoothly. In 2025, the ICH Assembly will take place on May 13-14 in Madrid, Spain. PhIRDA will continue to actively coordinate the participation of relevant working group experts in the ICH Assembly, deeply engaging in regulatory harmonization and mutual recognition efforts, contributing to the development of the global pharmaceutical industry.

 News & Events
News & Events
 2026-02-11
2026-02-11
 52
52

 News & Events
News & Events
 PHIRDA
PHIRDA  2026-01-19
2026-01-19
 99
99

 News & Events
News & Events
 PHIRDA
PHIRDA  2025-12-30
2025-12-30
 146
146