 News & Events
News & Events
 phirda
phirda  2024.06.26
2024.06.26
 386
386
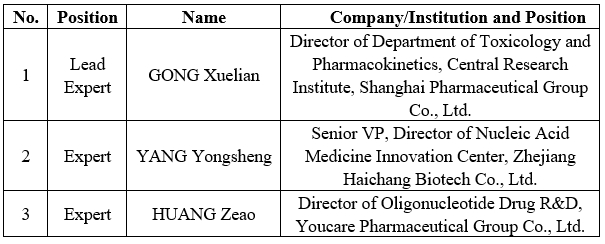
In March 2024, the Management Committee of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) decided to establish the S13: Nonclinical safety studies for Oligonucleotide-based Therapeutics Expert Working Group (EWG). Entrusted by the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA), China Pharmaceutical Innovation and Research Development Association (PhIRDA) successfully nominated three experts to participate in the IFPMA ICH S13 EWG, among which Gong Xuelian at the Shanghai Pharmaceutical Group Co., Ltd. was selected as the Lead Expert. She will lead the drafting of the concept paper of the Guideline and related work plans, and make positive contributions to the coordination and harmonisation of international rules in the field of non-clinical safety studies of oligonucleotide-based therapeutics. Below is the list of experts involved:
On June 4 this year, at the ICH Assembly held in Fukuoka, Japan, the National Medical Products Administration (NMPA), China, was elected to the ICH Management Committee for the third time. This demonstrates China’s alignment with international standards in new drug development and registration technology. It also highlights the international recognition of China’s progress in drug regulatory internationalization and significantly enhances China’s voice in international organizations. PhIRDA will continue to nominate experts from members to participate in the ICH WGs, deeply engaging in the harmonization of international regulatory rules, and promoting the implementation of ICH Guidelines in China. This will further integrate China’s pharmaceutical industry into the highest international standards system, contributing to the high-quality development of China’s pharmaceutical industry and global drug R&D and innovation.
As of June 2024, PhIRDA has successfully nominated 56 experts (including 11 Lead Experts, 9 Alternates) to 28 IFPMA ICH WGs, accounting for 40% of the global experts of IFPMA.
Click to see PhIRDA IFPMA ICH Expert List.

 News & Events
News & Events
 phirda
phirda  2024-08-14
2024-08-14
 91
91

 News & Events
News & Events
 phirda
phirda  2024-06-26
2024-06-26
 386
386

 News & Events
News & Events
 phirda
phirda  2024-06-25
2024-06-25
 269
269