 News & Events
News & Events
 phirda
phirda  2024.06.17
2024.06.17
 1733
1733
The Assembly of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) met in-person from June 1 to 5, 2024, in Fukuoka, Japan and relevant experts from 13 Working Groups (WGs) flied to Fukuoka to attend the Assembly. At this Assembly, the ICH welcomed ANMAT, Argentina and JFDA, Jordan as new ICH Members, bringing ICH to a total of 23 Members and 35 Observers (list attached).
A total of 13 WGs participated in the meeting. Three Leads of the ICH WGs nominated by the China Pharmaceutical Innovation and Research Development Association (PhIRDA), representing the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) attended in person. On behalf of IFPMA ICH M11, Q2(R2)/Q14, and Q6(R1) WGs respectively, they participated in the revision and discussion of relevant ICH Guidelines. Below is the list of WGs and experts involved:
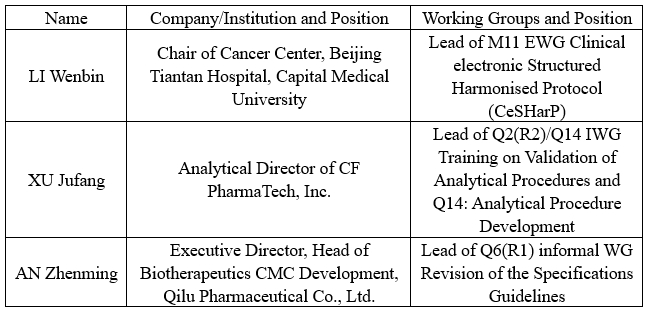
The three experts said that the ICH Assembly has achieved outstanding results, with each WG making significant progress: the M11 EWG revised some technical terms and has basically completed the document template, which is expected to enter the Step 3 of harmonisation in October this year; the Q2(R2)/Q14 IWG has completed the draft training materials, with the final version anticipated by the end of this year; the newly established Q6(R1) informal WG earlier this year has also completed the drafting of the concept paper and will begin drafting the Guideline this year. Three leads have diligently fulfilled their duties as a Lead by regularly organizing working group meetings and personally attending the annual ICH Assembly. Through active participation and coordination and promotion of various tasks, they have strengthened the exchanges and cooperation between the Chinese industry and international regulatory bodies and the industrial sector. Their efforts have facilitated the further integration of China’s pharmaceutical industry into the highest international regulatory frameworks, contributing to the advancement of pharmaceutical innovation in China and globally, the high-quality development of China’s pharmaceutical industry, and the fulfillment of medication needs for Chinese patients.

IFPMA delegates at the Assembly (from left: XU Jufang, third from right: LI Wenbin, sixth from right: AN Zhenming)

ICH Q2(R2)/Q14 IWG experts (XU Jufang, third from the right, first row)

ICH M11 EWG experts (LI Wenbin, second from left, third row)
As of June 2024, PhIRDA has nominated 53 experts to 27 IFPMA ICH WGs (including 10 Lead Experts, 8 Alternates), accounting for 40% of the global experts of IFPMA. PhIRDA has proactively nominated experts to participate in the IFPMA ICH WGs, facilitating direct involvement of Chinese experts in international rule-making processes. On behalf of PhIRDA and China’s pharmaceutical industry, these experts make the industry’s voice heard, and promote the implementation of ICH Guidelines in China. The next ICH Assembly meeting is planned on November 5 and 6 2024 in Montréal, Canada. PhIRDA will continue to actively coordinate the participation of relevant working group experts in the ICH Assembly, contributing to the formulation of international standards and enhancing the level of new drug development in China.
ICH Members & Observers
I. MEMBERS
1. Founding Regulatory Members
• EC, Europe
• FDA, United States
• MHLW/PMDA, Japan
2. Founding Industry Members
• EFPIA
• JPMA
• PhRMA
3. Standing Regulatory Members
• Health Canada, Canada
• Swissmedic, Switzerland
4. Regulatory Members
• ANVISA, Brazil
• ANMAT, Argentina
• COFEPRIS, Mexico
• EDA, Egypt
• HSA, Singapore
• JFDA, Jordan
• MFDS, Republic of Korea
• MHRA, UK
• NMPA, China
• SFDA, Saudi Arabia
• TFDA, Chinese Taipei
• TITCK, Türkiye
Industry Members
• BIO
• Global Self-Care Federation
• IGBA
II. OBSERVERS
1. Standing Observers
• IFPMA
• WHO
2. Legislative or Administrative Authorities
• AEC, Azerbaijan
• ANPP, Algeria
• CDSCO, India
• CECMED, Cuba
• CPED, Israel
• DPM, Tunisia
• Indonesian FDA, Indonesia
• INVIMA, Colombia
• MMDA, Moldova
• MOPH, Lebanon
• NAFDAC, Nigeria
• National Center, Kazakhstan
• NPRA, Malaysia
• NRA, Iran
• PPBHK, Hong Kong, China
• Roszdravnadzor, Russia
• SAHPRA, South Africa
• SCDMTE, Armenia
• SECMOH, Ukraine
• TGA, Australia
3. Regional Harmonisation Initiatives (RHIs)
• APEC
• ASEAN
• EAC
• GHC
• PANDRH
• SADC
4. International Pharmaceutical Industry Organisation
• APIC
5. International Organisation regulated or affected by ICH Guideline(s)
• Bill & Melinda Gates Foundation
• CIOMS
• EDQM
• IPEC
• PIC/S
• USP

 News & Events
News & Events
 PHIRDA
PHIRDA  2025-07-24
2025-07-24
 329
329

 News & Events
News & Events
 PHIRDA
PHIRDA  2025-07-09
2025-07-09
 258
258

 News & Events
News & Events
 2025-06-09
2025-06-09
 397
397