 News & Events
News & Events
 phirda
phirda  2024.03.29
2024.03.29
 1795
1795
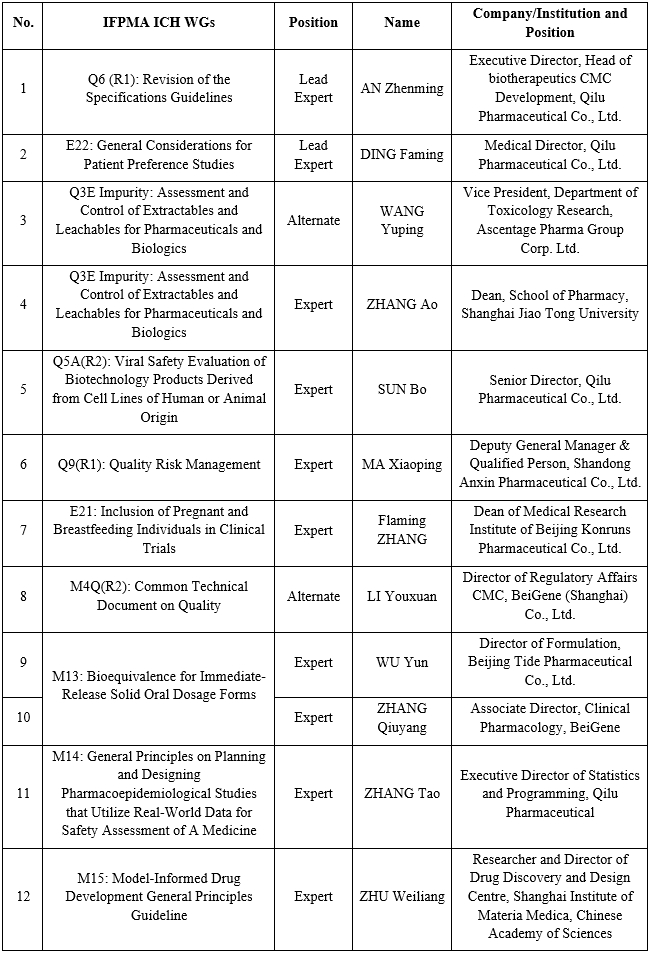
From Oct. 28 to Nov. 1, 2023, the ICH Assembly was held in Prague, during which several ICH Guidelines entered different steps. Entrusted by the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) after internal selection, China Pharmaceutical Innovation and Research Development Association (PhIRDA) has successfully nominated eight experts to several IFPMA ICH Working Groups (WGs). Among them, two experts were selected as the Lead Experts, leading the formulation, amendment and implementation of related ICH Guidelines. Besides, PhIRDA has successively nominated four experts to join the newly established ICH WGs, making positive contribution to the harmonisation of the international rules in related fields. Below is the list of ICH WGs and experts involved:
As of February 2024, PhIRDA has nominated 50 experts to 27 IFPMA ICH WGs (including 11 Lead Experts, 9 Alternates), accounting for 40% of the global experts of IFPMA. PhIRDA has proactively nominated experts to participate in the IFPMA ICH WGs, facilitating direct involvement of Chinese experts in international rule-making processes. On behalf of PhIRDA and the pharmaceutical industry in China, these experts make the industry’s voice heard, contribute Chinese wisdom, and promote the implementation of ICH Guidelines in China.
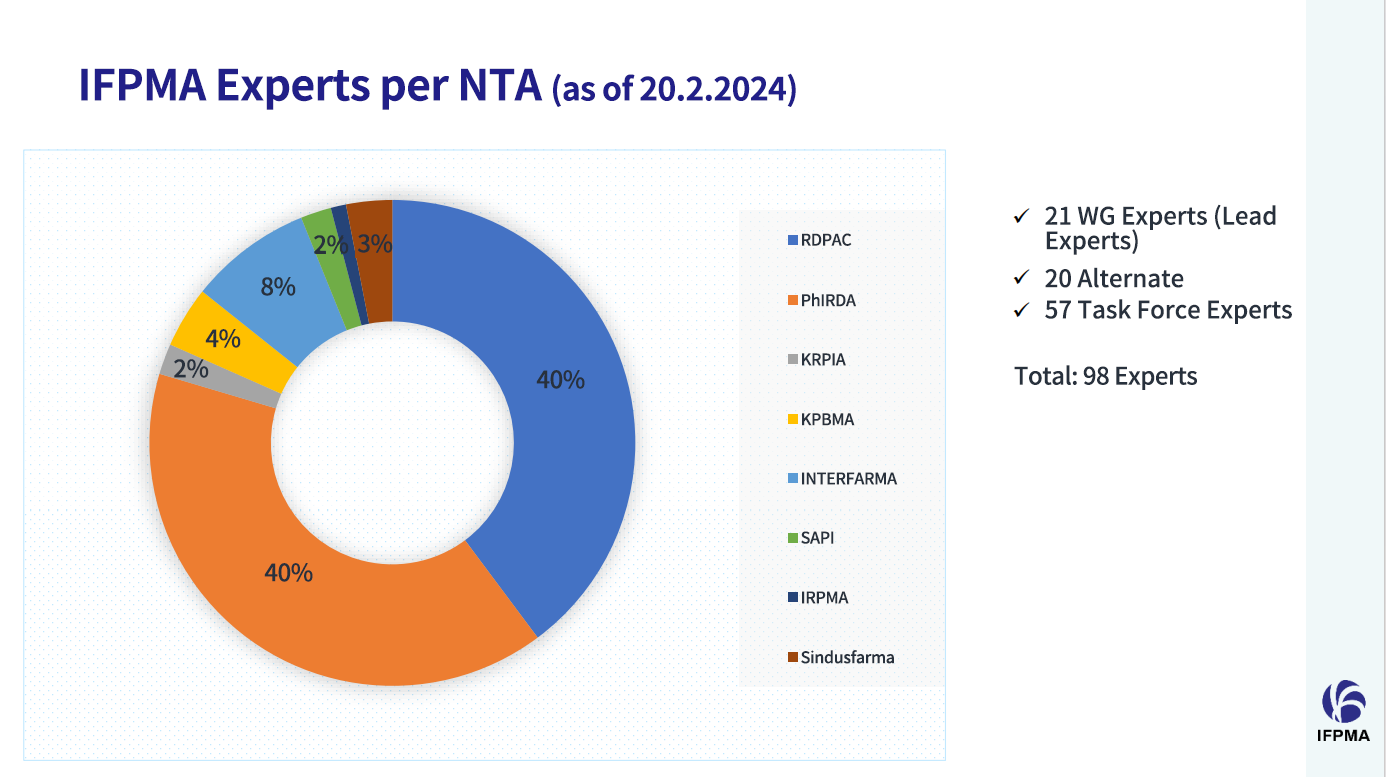
As of Feb. 2024, experts from PhIRDA account for 40 % of the global experts of IFPMA Source: IFPMA
Click to see PhIRDA IFPMA ICH Expert List

 News & Events
News & Events
 PHIRDA
PHIRDA  2025-12-22
2025-12-22
 6
6

 News & Events
News & Events
 PHIRDA
PHIRDA  2025-12-16
2025-12-16
 37
37

 News & Events
News & Events
 PHIRDA
PHIRDA  2025-12-09
2025-12-09
 68
68