 News & Events
News & Events
 phirda
phirda  2023.10.27
2023.10.27
 1456
1456
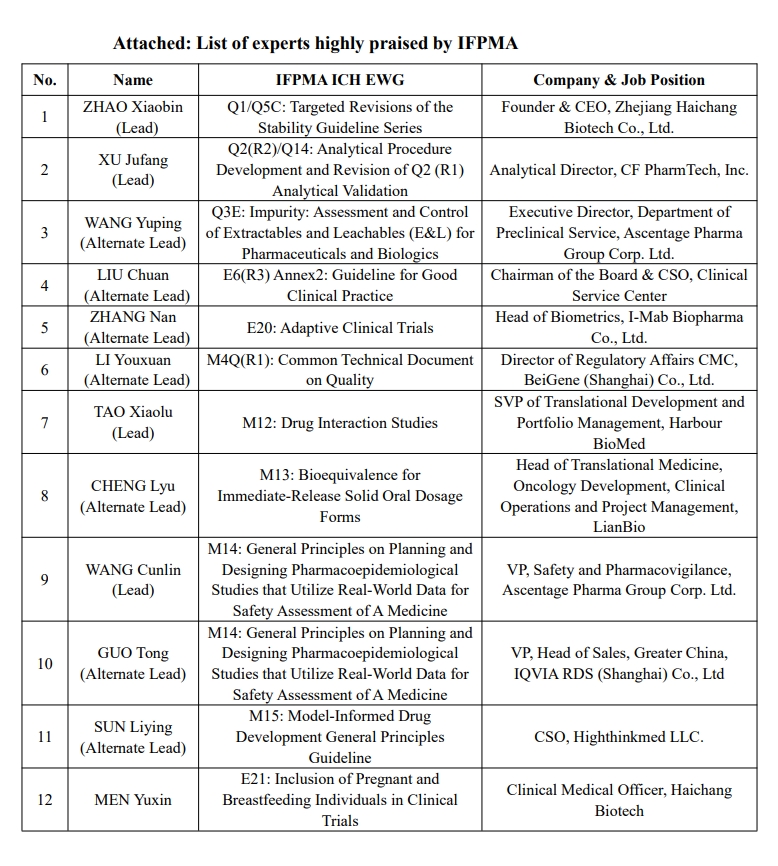
Since 2017, China Pharmaceutical Innovation and Investment Association (PhIRDA) has recommended many experts to participate in ICH Expert Working Groups (EWG). To date, 50 experts have been recommended to participate in 25 ICH EWGs, including 26 leads and alternates.
Recently, many experts (list attached) are highly affirmed and praised by the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) for their hard work in the harmonisation of the ICH Guidelines. He/She proactively fulfills his/her responsibilities, organizes/participates in regular ICH Working Group meetings, keeps in close contact with experts of working group and coordinators from IFPMA, gives feedback in a timely manner, and draft working report and summary. All these show that they actively participate in and promote all kinds of work. Their performance in ICH EWGs not only demonstrated the competence of professionals from the pharmaceutical industry in China, but also lays a solid foundation for PhIRDA to further engage in the IFPMA and ICH related work in the future. PhIRDA extends sincere thanks and gives high praise to these experts for their time, energy, and efforts in ICH harmonisation activities.
At the same time, PhIRDA extends thanks to members for recommending these experts and supporting them to participate in the international meetings. We wish PhIRDA’s members can recommend more experts with capability, responsibility, and commitment to engage in ICH related work, making China’s voice heard and contributing Chinese wisdom on the world stage.

 News & Events
News & Events
 PHIRDA
PHIRDA  2026-01-19
2026-01-19
 57
57

 News & Events
News & Events
 PHIRDA
PHIRDA  2025-12-30
2025-12-30
 111
111

 News & Events
News & Events
 PHIRDA
PHIRDA  2025-12-22
2025-12-22
 149
149