 News & Events
News & Events
 2018.04.02
2018.04.02
 1567
1567
Organized by Hong Kong Exchanges and Clearing Limited (HKEX), the HKEX Biotech Summit 2018 (hereinafter refer to as Summit) was successfully held at HKEX Connect Hall on March 22, 2018. The Summit is dedicated to industry professionals to network, exchange insights and experience, and work together to promote the biotech funding ecosystem in Hong Kong. China Pharmaceutical Innovation and Research Development Association (hereinafter refer to as PhIRDA) organized over 40 represents from more than 20 of its members to attend the Conference. More than 600 biotech companies and industry organization managers, investors, analysts and other market participants gathered to have a deep discussion on the situation of global biotech development, the latest trend and challenges of Chinese biotech industry in the future.
Chung Kong CHOW, Chairman of HKEX Group, Paul CHAN, Financial Secretary of the Government of the Hong Kong Special Administrative Region, Carlson TONG, Chairman of Securities and Futures Commission, Ashley ALDER, Chief Executive Officer of Securities and Futures Commission and Charles LI, Chief Executive of HKEX Group addressed during the opening ceremony.

VIP Guests made opening speeches
Ruyi HE, Chief Scientist of China Food and Drug Administration (CFDA) Center for Drug Evaluation made a detailed introduction on how to facilitate drug review regulation and approval process. The year of 2017 marks an important period of China’s Pharmaceutical Innovation Development. After becoming the official member of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), CFDA has already started the relevant work of implementation of the ICH Tripartite Guidelines to bring the Chinese Pharmaceutical Safety in line with International working standards. Opinions on Deepening the Review and Approval System Reform and Encouraging the Drug and Medical Device Innovation issued by General Office of the CPC Central Committee and General Office of the State Council in October 2017 not only pointed out the right direction but also improved the quality and effect of Chinese Pharmaceutical Review and Approval System Reform.

Ruyi He made keynote speech
Ruilin SONG, Executive President of China Pharmaceutical Innovation and Research Development Association (PhIRDA) gave a keynote speech on the Development of Biotech Industry in China: Looking into the Future. Ruilin SONG said that the development pattern of China’s economy has transformed from high speed to high quality development, and biotech industry played an important role during the transformation. The relevant policies issued by the government have promoted the rapid growth of investment and financing in China's biotech industry. China's pharmaceutical R&D companies and investment institutions in the pharmaceutical sector have increased in the world market. VC/PE financing in the medical and health field is active, and mainland pharmaceutical companies are listed overseas. The trend is becoming more obvious, some innovative companies also have very bright performance on Nasdaq. After CFDA becoming the official member of ICH, China's drug review and approval standards will go in line with the international working standards. HKEX's policy for pre-revenue biotech companies’ IPO will undoubtedly bring a timely spring rain to the Chinese pharmaceutical industry.
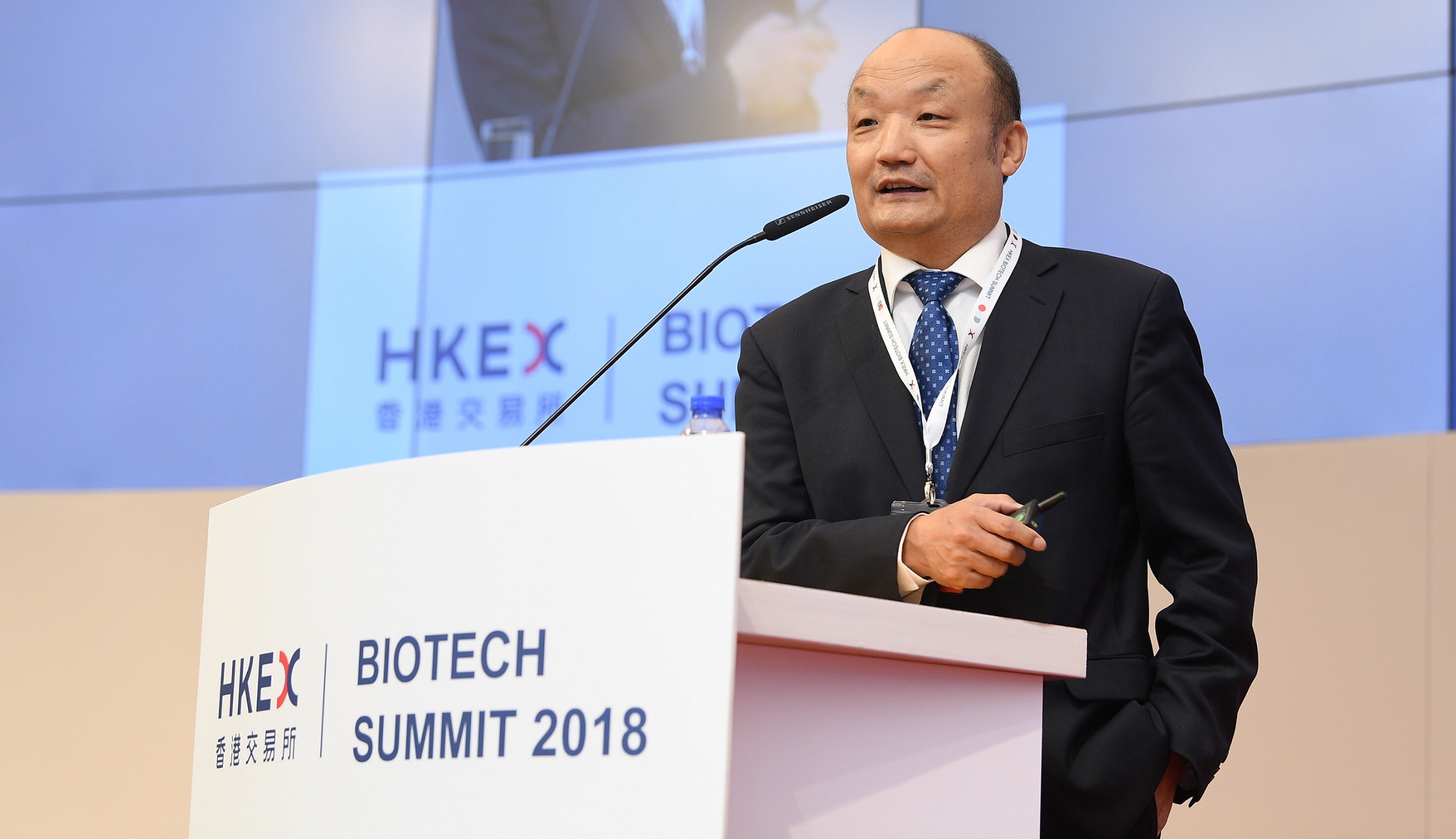
Ruilin SONG made keynote speech
Invited by HKEX, PhIRDA organized a panel discussion on the innovation and development trend of biotech industry in China. Lawrence TIAN, Founding Partner of YuanMing Capital and Vice Chairman of PhIRDA Pharmarceutical Innovation Investment Specialty Committee hosted the panel, Michael YU, Chairman and CEO of Innovent Biologics and Vice Chairman of PhIRDA Drug R&D Specialty Committee, Dajun YANG, Chairman and CEO of Ascentage Pharma and Vice Chairman of PhIRDA Drug R&D Specialty Committee and Ting XU, President and CEO of Alphamab made a discussion on the importance, characteristics of biotech industry in China, challenges and risks in the development of biotech companies and the differences between China and American biotech industry.

Panel Discussion Organized by PhIRDA

Plenary meeting
Although increasing number ofbiotech R&D companies in China could not profit immediately, the return on equity is rising in high speed. HKEX has published a consultation paper seeking public feedback on the proposed new rules to accommodate the listings of companies from emerging and innovative sectors, with a new chapter focusing on the listing of pre-revenue biotech issuers. The proposed new chapter will widen market access for these companies to create a more vibrant marketplace in Hong Kong.

 News & Events
News & Events
 PHIRDA
PHIRDA  2025-12-16
2025-12-16
 12
12

 News & Events
News & Events
 PHIRDA
PHIRDA  2025-12-09
2025-12-09
 46
46

 News & Events
News & Events
 PHIRDA
PHIRDA  2025-07-24
2025-07-24
 588
588